Electroplating in jewelry making involves coating a metal surface with a thin layer of another metal through an electric current, providing enhanced durability and a rich, lustrous finish. Anodizing, primarily used on aluminum, creates a protective oxide layer by electrochemical oxidation, offering vibrant color options and increased corrosion resistance without altering the metal's natural texture. Choosing between electroplating and anodizing depends on the base metal, desired appearance, and longevity requirements of the jewelry piece.
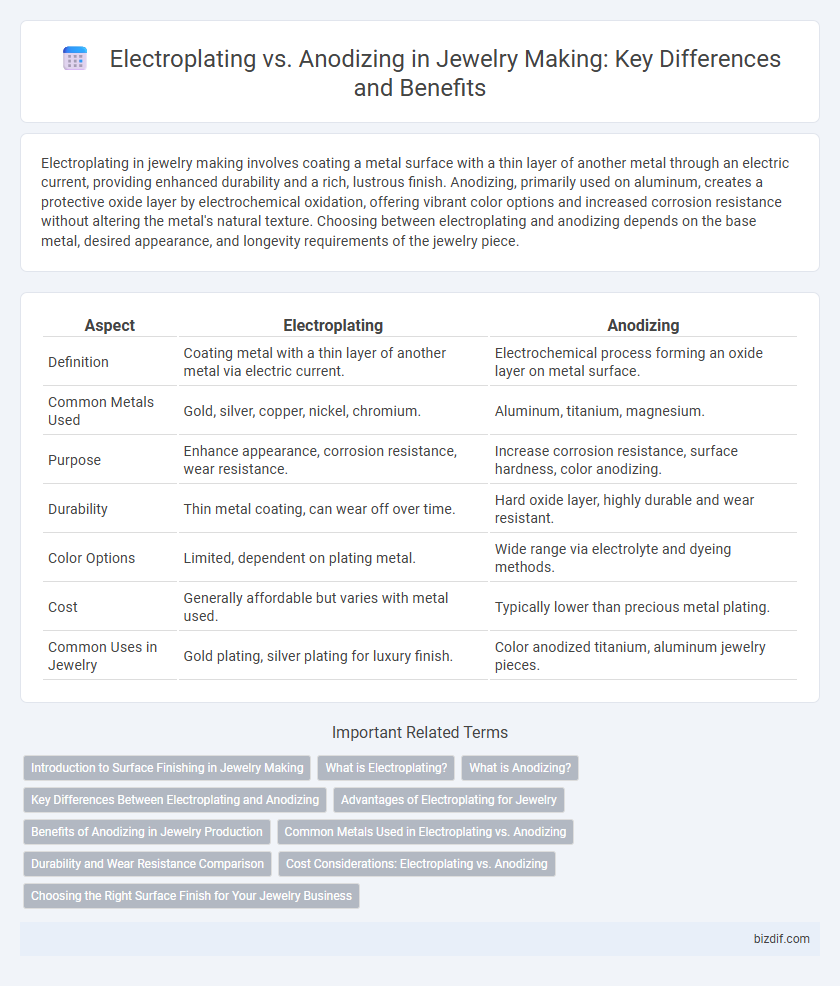
Table of Comparison
| Aspect | Electroplating | Anodizing |
|---|---|---|
| Definition | Coating metal with a thin layer of another metal via electric current. | Electrochemical process forming an oxide layer on metal surface. |
| Common Metals Used | Gold, silver, copper, nickel, chromium. | Aluminum, titanium, magnesium. |
| Purpose | Enhance appearance, corrosion resistance, wear resistance. | Increase corrosion resistance, surface hardness, color anodizing. |
| Durability | Thin metal coating, can wear off over time. | Hard oxide layer, highly durable and wear resistant. |
| Color Options | Limited, dependent on plating metal. | Wide range via electrolyte and dyeing methods. |
| Cost | Generally affordable but varies with metal used. | Typically lower than precious metal plating. |
| Common Uses in Jewelry | Gold plating, silver plating for luxury finish. | Color anodized titanium, aluminum jewelry pieces. |
Introduction to Surface Finishing in Jewelry Making
Electroplating in jewelry making involves depositing a thin layer of metal, such as gold or silver, onto the surface of a base metal to enhance durability and aesthetic appeal. Anodizing, primarily used with aluminum jewelry, creates a protective oxide layer that can be dyed in various colors, offering both corrosion resistance and unique visual effects. Surface finishing techniques like electroplating and anodizing play a crucial role in protecting jewelry from wear while enhancing its design and longevity.
What is Electroplating?
Electroplating is a jewelry-making process that uses an electric current to deposit a thin layer of metal, such as gold, silver, or rhodium, onto the surface of a base metal, enhancing its appearance and resistance to corrosion. This technique improves durability and provides a uniform, lustrous finish that can mimic the look of solid precious metals at a lower cost. Electroplated jewelry is widely used for affordable luxury, allowing intricate designs to maintain their shine and color over time.
What is Anodizing?
Anodizing is an electrochemical process that enhances the surface of metal jewelry by forming a protective oxide layer, primarily on aluminum alloys. This technique increases corrosion resistance and allows for vibrant, long-lasting color options without the use of dyes or paints. Anodized jewelry benefits from improved durability and aesthetic appeal, making it a popular choice for lightweight, colorful designs.
Key Differences Between Electroplating and Anodizing
Electroplating involves depositing a thin layer of metal such as gold, silver, or nickel onto jewelry surfaces to enhance appearance and prevent corrosion, while anodizing is an electrochemical process primarily used on aluminum that thickens the natural oxide layer for increased durability and color customization. Electroplating typically offers a glossy, metallic finish with potential variations depending on the plating metal, whereas anodizing provides a more matte or satin finish with vibrant, integrated color options due to dye absorption in the oxide layer. Key differences lie in materials compatibility, with electroplating applicable to a broader range of metals, and anodizing limited to aluminum and its alloys, alongside distinctions in corrosion resistance, wear properties, and environmental impact.
Advantages of Electroplating for Jewelry
Electroplating enhances jewelry durability by providing a uniform, corrosion-resistant metallic coating that improves wear resistance and prevents tarnishing. It allows for precise control over thickness and finish, enabling the application of precious metals like gold or rhodium at an affordable cost. The process also offers superior conductivity and adhesion, ensuring the plating remains intact during daily use.
Benefits of Anodizing in Jewelry Production
Anodizing enhances jewelry durability by creating a protective oxide layer that resists corrosion and wear, unlike electroplating which relies on thin metal coatings prone to chipping. This process allows for vibrant, long-lasting color options without adding significant thickness, preserving intricate details in designs. Anodizing is also more environmentally friendly, eliminating the use of hazardous chemicals typical in electroplating, making it a sustainable choice in jewelry production.
Common Metals Used in Electroplating vs. Anodizing
Electroplating commonly uses metals such as copper, nickel, gold, and silver to coat base metals like brass and steel for enhanced corrosion resistance and aesthetic appeal. Anodizing, primarily applied to aluminum, titanium, and magnesium, creates a durable oxide layer that improves hardness and allows for vibrant dyeing options. Understanding the differences in base metals and coating techniques is crucial for selecting the ideal finishing process in jewelry making.
Durability and Wear Resistance Comparison
Electroplating deposits a thin layer of metal such as gold, silver, or rhodium onto jewelry surfaces, offering excellent corrosion resistance but potentially wearing off with prolonged abrasion. Anodizing, primarily used on aluminum, forms a durable oxide layer bonded to the metal, significantly enhancing scratch resistance and wear durability without peeling. For jewelry subjected to frequent contact and friction, anodized pieces generally maintain their appearance longer, while electroplated finishes may require more frequent reapplication to sustain protection.
Cost Considerations: Electroplating vs. Anodizing
Electroplating typically involves higher material and labor costs due to expensive metal coatings such as gold or silver and intricate surface preparation processes. Anodizing offers a more cost-effective solution primarily for aluminum-based jewelry, using a less costly electrochemical oxidization process that provides durable color finishes. Choosing between electroplating and anodizing depends on budget constraints, desired finish quality, and metal type compatibility in jewelry production.
Choosing the Right Surface Finish for Your Jewelry Business
Electroplating enhances jewelry by depositing a thin layer of metal, such as gold or silver, providing a durable, glossy finish that resists tarnish and adds value. Anodizing primarily applies to aluminum jewelry, creating a colorful, corrosion-resistant oxide layer that is both decorative and long-lasting. Selecting between electroplating and anodizing depends on factors like metal type, desired aesthetics, durability, and customer preferences within your jewelry business.
Electroplating vs anodizing Infographic
 bizdif.com
bizdif.com