Saponification is the chemical reaction where fats or oils react with an alkali, producing soap and glycerin, ideal for creating natural pet soaps that gently clean and moisturize fur. Neutralization occurs when excess alkali or acid is balanced, ensuring the soap is mild and safe for pets' sensitive skin. Understanding these processes helps formulate effective, gentle pet soaps that maintain optimal pH levels for healthy coats.
Table of Comparison
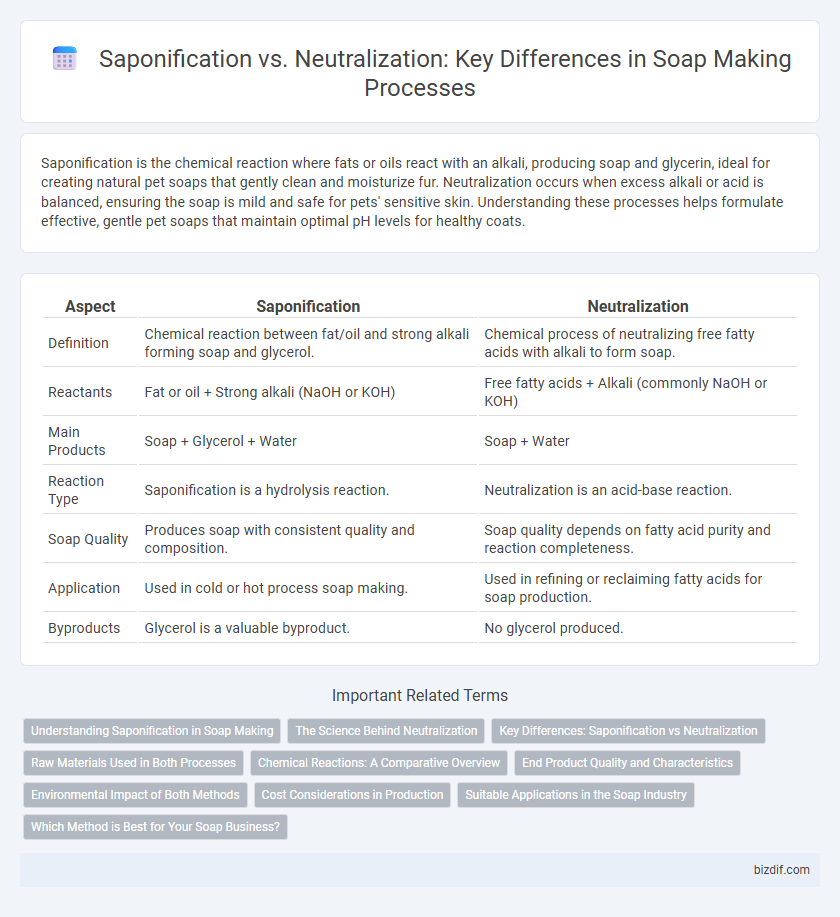
| Aspect | Saponification | Neutralization |
|---|---|---|
| Definition | Chemical reaction between fat/oil and strong alkali forming soap and glycerol. | Chemical process of neutralizing free fatty acids with alkali to form soap. |
| Reactants | Fat or oil + Strong alkali (NaOH or KOH) | Free fatty acids + Alkali (commonly NaOH or KOH) |
| Main Products | Soap + Glycerol + Water | Soap + Water |
| Reaction Type | Saponification is a hydrolysis reaction. | Neutralization is an acid-base reaction. |
| Soap Quality | Produces soap with consistent quality and composition. | Soap quality depends on fatty acid purity and reaction completeness. |
| Application | Used in cold or hot process soap making. | Used in refining or reclaiming fatty acids for soap production. |
| Byproducts | Glycerol is a valuable byproduct. | No glycerol produced. |
Understanding Saponification in Soap Making
Saponification is the chemical reaction between fats or oils and a strong alkali, typically sodium hydroxide, producing soap and glycerin. This process transforms triglycerides into soap molecules through hydrolysis, which is essential for creating solid soap bars. Unlike neutralization, which involves balancing acids and bases without forming soap, saponification specifically generates soap as a product, impacting the texture and cleansing properties of the final product.
The Science Behind Neutralization
The science behind neutralization in soap making involves the chemical reaction where free alkali in the soap is balanced by the addition of acids, ensuring the final product is mild and safe for skin. Neutralization stabilizes the soap's pH, preventing harshness and improving texture compared to incomplete saponification reactions. This precise control of pH through neutralization is essential for producing high-quality, skin-friendly soap bars.
Key Differences: Saponification vs Neutralization
Saponification is a chemical reaction where fats or oils react with an alkali, typically sodium hydroxide or potassium hydroxide, to produce soap and glycerin, essential in traditional soap making. Neutralization involves adjusting the pH of a soap solution by adding acid or alkali to stabilize the product and improve skin compatibility, without forming new soap molecules. The key difference lies in saponification generating soap from raw materials, while neutralization modifies the pH of an already formed soap solution.
Raw Materials Used in Both Processes
Saponification uses triglyceride fats or oils, such as coconut oil, olive oil, or palm oil, reacted with a strong alkali like sodium hydroxide (NaOH) or potassium hydroxide (KOH) to produce soap and glycerol. Neutralization involves the reaction of free fatty acids with alkalis, commonly sodium hydroxide, to form soap, often starting with fatty acid-rich byproducts like tallow or acidulated soapstock. Both processes require precise raw materials selection to ensure soap quality, with saponification relying on whole fats and neutralization focusing on free fatty acid feedstocks.
Chemical Reactions: A Comparative Overview
Saponification is a chemical reaction where triglycerides in fats or oils react with a strong alkali, such as sodium hydroxide, producing glycerol and soap molecules, typically fatty acid salts. Neutralization involves the reaction of free fatty acids with a base to form soap, aimed at reducing acidity levels and improving soap quality. Both processes are essential in soap making, with saponification converting oils into soap and neutralization refining the final product by eliminating excess acids.
End Product Quality and Characteristics
Saponification produces soap by chemically reacting fats or oils with an alkali, resulting in a product with natural moisturizing properties, better lather, and a longer shelf life. Neutralization involves adjusting the pH of acidic or basic solutions to form salts, which may lack the moisturizing benefits and cleansing power of saponified soaps. End products from saponification typically exhibit superior skin compatibility and durability compared to those formed via neutralization.
Environmental Impact of Both Methods
Saponification, the chemical reaction between fats and alkali producing soap and glycerin, generates biodegradable products with low environmental toxicity, making it an eco-friendly choice in soap making. Neutralization, involving the reaction of fatty acids with alkali to form soap salts, often uses synthetic acids or bases, which can result in higher chemical waste and potential water pollution. Saponification typically results in less hazardous effluent and reduced environmental impact compared to neutralization processes.
Cost Considerations in Production
Saponification involves the chemical reaction between fats and lye, producing soap and glycerin with minimal additional costs, making it a cost-effective method in soap production. Neutralization requires precise acid-base balance and often involves added costs for acid or alkali adjustments, impacting overall production expenses. Choosing saponification over neutralization generally reduces raw material and processing costs, optimizing budget efficiency in large-scale soap manufacturing.
Suitable Applications in the Soap Industry
Saponification is ideal for producing traditional solid soaps with strong cleansing properties, as it involves the chemical reaction between fats and lye to create soap and glycerin. Neutralization suits liquid soap manufacturing where controlling pH is crucial, using acids to neutralize excess alkali and achieve a milder product suitable for sensitive skin. Both processes are essential in the soap industry, with saponification preferred for bar soaps and neutralization favored in gentle liquid or transparent soap formulations.
Which Method is Best for Your Soap Business?
Saponification is the chemical process where fats or oils react with lye to produce soap and glycerin, offering natural cleansing properties and a moisturizing effect that appeals to eco-conscious consumers. Neutralization involves adjusting the pH of soap to make it milder and less irritating, often used for sensitive skin products but can add complexity and cost to production. For a soap business, saponification provides a straightforward and authentic approach with natural benefits, while neutralization is best suited for creating specialty soaps requiring precise pH control for targeted skin care needs.
Saponification vs Neutralization Infographic
 bizdif.com
bizdif.com