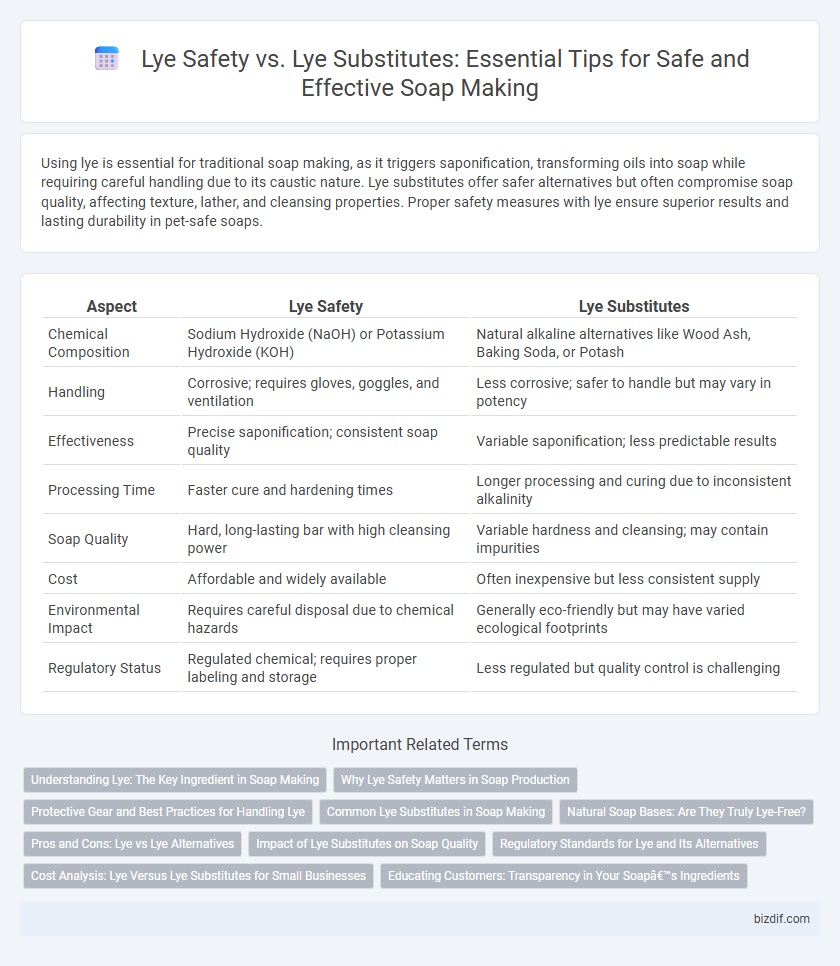
Using lye is essential for traditional soap making, as it triggers saponification, transforming oils into soap while requiring careful handling due to its caustic nature. Lye substitutes offer safer alternatives but often compromise soap quality, affecting texture, lather, and cleansing properties. Proper safety measures with lye ensure superior results and lasting durability in pet-safe soaps.
Table of Comparison
| Aspect | Lye Safety | Lye Substitutes |
|---|---|---|
| Chemical Composition | Sodium Hydroxide (NaOH) or Potassium Hydroxide (KOH) | Natural alkaline alternatives like Wood Ash, Baking Soda, or Potash |
| Handling | Corrosive; requires gloves, goggles, and ventilation | Less corrosive; safer to handle but may vary in potency |
| Effectiveness | Precise saponification; consistent soap quality | Variable saponification; less predictable results |
| Processing Time | Faster cure and hardening times | Longer processing and curing due to inconsistent alkalinity |
| Soap Quality | Hard, long-lasting bar with high cleansing power | Variable hardness and cleansing; may contain impurities |
| Cost | Affordable and widely available | Often inexpensive but less consistent supply |
| Environmental Impact | Requires careful disposal due to chemical hazards | Generally eco-friendly but may have varied ecological footprints |
| Regulatory Status | Regulated chemical; requires proper labeling and storage | Less regulated but quality control is challenging |
Understanding Lye: The Key Ingredient in Soap Making
Lye, or sodium hydroxide, is the essential alkaline agent in traditional soap making, causing saponification by reacting with fats and oils to create soap molecules. Understanding lye's chemical properties and handling precautions ensures safe and effective soap production, as its caustic nature can cause burns and requires precise measurement for balanced results. While lye substitutes may seem safer, they often compromise soap quality, texture, and cleansing abilities, making genuine lye irreplaceable for authentic soap craftsmanship.
Why Lye Safety Matters in Soap Production
Proper lye safety in soap production is crucial due to the caustic nature of sodium hydroxide, which can cause severe chemical burns and respiratory hazards if mishandled. Using protective gear such as gloves, goggles, and ventilation systems minimizes risks during the saponification process. Substitutes often lack the efficacy of traditional lye, making safe handling protocols indispensable for achieving high-quality, consistent soap products.
Protective Gear and Best Practices for Handling Lye
Wearing chemical-resistant gloves, safety goggles, and long sleeves is essential when handling lye to prevent severe chemical burns and eye injuries. Best practices include working in a well-ventilated area, slowly adding lye to water to avoid splashing, and never substituting lye with alternative substances like baking soda or washing soda, as these do not saponify oils properly. Proper storage in clearly labeled, airtight containers and having vinegar nearby to neutralize spills further enhances safety during soap making.
Common Lye Substitutes in Soap Making
Common lye substitutes in soap making include potassium hydroxide (KOH) and sodium hydroxide (NaOH), with KOH typically used for liquid soaps and NaOH for solid bars. While these substitutes serve similar roles in saponification, potassium hydroxide provides a gentler alternative suitable for softer soaps and less skin irritation. Proper handling and accurate measurements remain critical to avoid soap failure and ensure user safety despite substituting traditional lye sources.
Natural Soap Bases: Are They Truly Lye-Free?
Natural soap bases often claim to be lye-free, but true soap making requires a chemical reaction called saponification, which involves lye (sodium hydroxide or potassium hydroxide). Lye substitutes may compromise soap quality, texture, or safety, as improper alternatives can result in incomplete saponification or harsh residues. Understanding that soap bases labeled "lye-free" generally use pre-saponified ingredients helps consumers avoid confusion while ensuring safe, effective soap products.
Pros and Cons: Lye vs Lye Alternatives
Lye, chemically known as sodium hydroxide, is essential for saponification, yielding durable and high-quality soaps but requiring careful handling due to its caustic nature and potential skin burns. Lye substitutes, such as potash (potassium hydroxide) or commercial melt-and-pour bases, offer safer handling and convenience but often result in softer soaps or less control over the soap's final properties. Soap makers must balance the precision and traditional results of using lye with the ease and safety offered by lye alternatives, depending on their skill level and soap type desired.
Impact of Lye Substitutes on Soap Quality
Lye substitutes often lack the strong alkaline properties required for proper saponification, resulting in softer, less durable soap bars. These alternatives can lead to incomplete chemical reactions, causing uneven texture and reduced cleansing effectiveness. Maintaining traditional lye use ensures consistent soap quality, firmness, and long-lasting usability.
Regulatory Standards for Lye and Its Alternatives
Regulatory standards for lye (sodium hydroxide) in soap making strictly mandate labeling, handling, and storage requirements to ensure user safety and compliance with government agencies like OSHA and the EPA. Lye substitutes such as potassium hydroxide or organic alternatives often face differing regulatory scrutiny, with some formulations exempt from stringent industrial hazardous chemical classifications but still subject to consumer safety laws. Understanding these regulations helps soap makers choose compliant ingredients while maintaining effective saponification and product quality.
Cost Analysis: Lye Versus Lye Substitutes for Small Businesses
Lye remains the most cost-effective ingredient for small soap businesses due to its low price and high availability compared to lye substitutes such as potassium hydroxide or natural alkalis, which often incur higher purchase and shipping costs. Despite safety concerns, proper handling and storage of lye can minimize risks without the added expense of specialized substitutes that may alter soap quality and production efficiency. Small-scale soap makers must weigh lye's affordability and consistent saponification performance against the premium cost and variability of lye alternatives when evaluating overall production expenses.
Educating Customers: Transparency in Your Soap’s Ingredients
Transparent communication about lye safety and its substitutes is essential for educating customers on soap-making ingredients. Clear labeling of sodium hydroxide or potassium hydroxide highlights the importance of proper handling and curing times, while explaining natural alternatives like soapwort or castile soap supports informed choices. Emphasizing ingredient transparency fosters trust and helps customers understand the impact of lye on soap quality and safety.
Lye Safety vs Lye Substitutes Infographic
 bizdif.com
bizdif.com